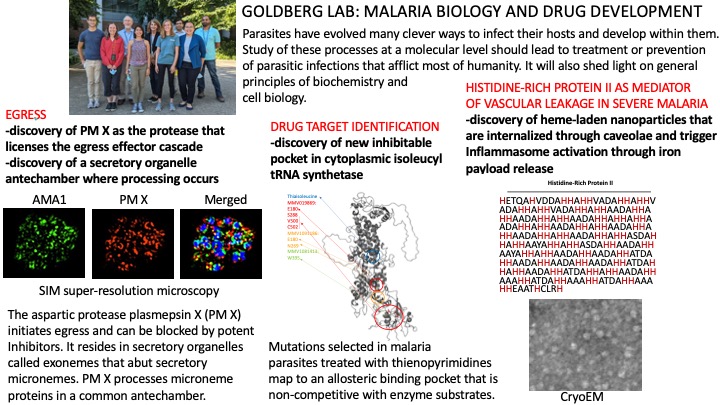
Parasites have evolved many clever ways to infect their hosts and develop within them. Study of these processes at a molecular level should lead to treatment or prevention of parasitic infections that afflict most of humanity. It will also shed light on general principles of biochemistry and cell biology. The organism we are studying is Plasmodium falciparum, a protozoan parasite that causes malaria.
Intraerythrocytic malaria parasites are fascinating creatures. They have evolved many clever strategies for survival inside their host cell. Most of these adaptations are still biological mysteries. Indeed, nearly half of the Plasmodium proteome comprises proteins of unknown function. Some are species or phylum specific; others have orthologs spread widely through phylogeny. We are interested in defining the roles of such proteins, with a special focus on proteases of unknown function. We use a combination of genetic and biochemical approaches to elucidate biological roles. To help us with this analysis, we have developed regulated protein knockdown systems for the study of essential proteins. This has allowed us to define a role for Plasmodium calpain in nucleolar control of cell cycle progression,a role for the aspartic protease plasmepsin V in export of parasite proteins to the host erythrocyte, and roles for plasmepsins IX and X in invasion/egress.
We are particularly interested in exported proteins. The parasite exports several hundred proteins into its host erythrocyte. What are these proteins doing in the host cell and beyond? How do the proteins get out of the parasite?
Another mysterious attribute of the Plasmodium proteome is that nearly one-third of all proteins have runs of asparagine. Two proteins we are studying have 30 and 80 consecutive asparagines. What are these asparagines for? Since polyasparagine is amyloidogenic, how does the cell handle all these proteins? Using genetic and biochemical manipulations, we are trying to address such issues.
Plasmodium has more than 5,000 predicted gene products. New drug targets are desperately needed, but which genes are essential? We are using approaches such as allelic replacement and chemical genetics to get at this question. One focus is isoleucine utilization. We have found that isoleucine is the sole exogenous amino acid required for parasite growth. By selecting parasites resistant to isoleucine utilization inhibitors, we have been able to define targets within this pathway. Whole genome sequencing can pinpoint resistance mutations. Allelic replacement then allows us to show that a given mutation in a target is responsible for the observed resistance.
Our work involves a combination of biochemical, genetic, genomic, cell biological, and physiological approaches aimed at understanding the biology of this nefarious organism.